Abstract
On behalf of the European MCL Network
Introduction: Rituximab (R) maintenance after immunochemotherapy represents standard of care in older mantle cell lymphoma (MCL) patients. The European MCL Elderly trial has shown the efficacy of R maintenance after R-CHOP in terms of PFS and OS (Kluin-Nelemans et al., JCO 2020). Induction with R-FC showed similar response rates and failure-free survival but inferior OS as compared to R-CHOP. The assessment of minimal residual disease (MRD) represents a promising tool for individualized treatment decisions. Within the European MCL Elderly trial, we investigated whether MRD status predicts the efficacy of maintenance and aimed to identify MRD-based trigger time points for targeted intervention.
Patients and methods: The MCL Elderly trial included patients with previously untreated, advanced stage MCL aged 60 years or older and not suitable for autologous stem cell transplantation. MRD assessment was done at predefined time points during and after treatment until clinical progression for patients recruited in France or Germany with baseline samples and at least one follow-up sample. Three central MRD labs performed marker screening (BCL1-JH t(11;14) translocation or IGH rearrangement) and real-time quantitative PCR (qPCR) following standardized ESG guidelines, as previously reported (Pott et al., Blood 2010). MRD status was pooled from bone marrow and peripheral blood results by classifying a patient as MRD negative if all available samples at the respective time point were negative. Predictive value of MRD status at end of induction for efficacy of maintenance was analysed with Cox regression for PFS including the interaction term of MRD status and treatment group. To assess the prognostic value of MRD status for clinical progression, landmarks were defined at midterm induction (MI), end of induction (EOI), and during four 6-months periods following EOI.
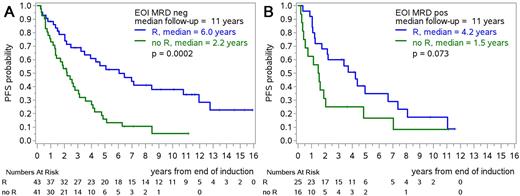
Results: A molecular marker for MRD assessment was generated in 85% of 288 screened patients and in 80% a qPCR assay fulfilling standardized criteria could be established. Patients in whom a molecular marker could be identified had more frequently generalized disease compared with those without molecular marker (85% vs. 51% stage IV, 78% vs. 37% with bone marrow involvement, median LDH/ULN quotient: 0.93 vs. 0.81). Among patients without clinical progression during induction, MRD-negativity was achieved more frequently and earlier with R-FC (MI: 63% of 82 patients; EOI: 80% of 82) than with R-CHOP (MI: 26% of 84, p<0.0001; EOI: 53% of 89, p=0.0002). The efficacy of maintenance was clearly seen in patients MRD negative at EOI (PFS-HR 0.38, 95% CI 0.21-0.63, Figure 1A) and was slightly less pronounced in MRD positive patients (HR 0.51, 95% CI 0.26-1.02; interaction p=0.48, Figure 1B). Especially after R-CHOP, the efficacy of R-maintenance was higher among MRD-negative patients (HR 0.23, 95% CI 0.10-0.52) than among MRD-positive patients (HR 0.59, 95% 0.28-1.26, interaction p=0.097). Landmark analyses confirmed the prognostic value of MRD status during and after R-FC and during maintenance/follow-up after R-CHOP. Median time from MRD-positivity 6/12/18/24 months from EOI to clinical progression was consistently short with 2.2/1.2/1.6/1.4 years after R-CHOP and 1.0/1.0/0.7/0.7 years after R-FC.
Conclusions: The results confirm the strong efficacy of R-maintenance in MRD-negative patients after induction. Omitting or stopping maintenance based on MRD-negativity is thus discouraged. Considering the short time to progression, more effective treatment strategies should be explored in MRD-positive patients to improve the long-term prognosis of patients with MCL.
Disclosures
Delfau:Roche: Research Funding; Celgene: Research Funding; Gilead: Honoraria; Amgen: Honoraria; Mundipharma: Other. Stilgenbauer:Hoffmann-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZenica: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Infinity: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sunesis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Salles:Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys AG, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol-Myers Squibb, BeiGene, Incyte, Miltenyi Biotec, Ipsen, Kite, a Gilead Company, Loxo, Rapt: Consultancy; AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, a Gilead Company, Miltenyi, MorphoSys, Takeda, and VelosBio: Membership on an entity's Board of Directors or advisory committees; Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys AG, Amgen, Bayer, Epizyme, Regeneron, Kite, a Gilead Company: Honoraria. Thieblemont:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Hospira: Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Bayer: Honoraria; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding. Tilly:Incyte: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kanz:Curevac: Current equity holder in private company. Feugier:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Huebel:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; Sanofi: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; EUSA: Consultancy, Speakers Bureau; AbbVie: Consultancy; Novartis: Consultancy, Speakers Bureau; Servier: Research Funding; Janssen: Research Funding; Beigene: Speakers Bureau. Schmidt:Bayer Healthcare: Research Funding; Janssen: Other: Travel Support; Kite Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support. Ribrag:Incyte: Membership on an entity's Board of Directors or advisory committees; Pharmamar: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Nanostring: Membership on an entity's Board of Directors or advisory committees; Infinity: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; AZ: Membership on an entity's Board of Directors or advisory committees; Abbvie: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Epizyme: Research Funding; GSK: Research Funding; Astex: Research Funding. Dreyling:Amgen, Astra Zeneca, Gilead/Kite, Janssen, Lilly, Novartis, Roche: Honoraria; Abbvie, Bayer, BMS/Celgene, Gilead/Kite, Janssen, Roche: Research Funding; Astra Zeneca, Beigene, BMS/Celgene, Gilead/Kite, Janssen, Lilly/Loxo, Novartis, Roche: Consultancy.
OffLabel Disclosure:
Rituximab maintenance in mantle cell lymphoma
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal